Gay-Lussac's Law Calculator
This tool will calculate any parameter from the equation defined by Gay-Lussac's law p1/t1=p2/t2
To Calculate :
Pressure 1:
Temperature 1:
Pressure 2:
Temperature 2:
Result
x
How to use this Gay-Lussac's Law Calculator Tool?
How to use Yttags's Gay-Lussac's Law Calculator?
- Step 1: Select the Tool

- Step 2: Enter The Following Options And Click On Calculate Button
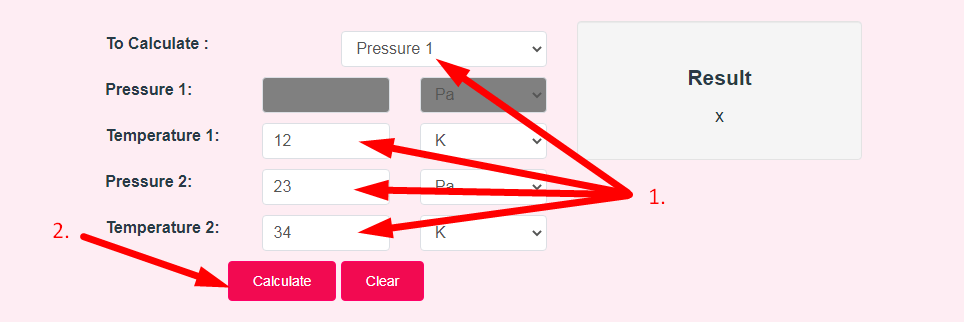
- Step 3: Check Your Gay-Lussac's Law Calculator Result
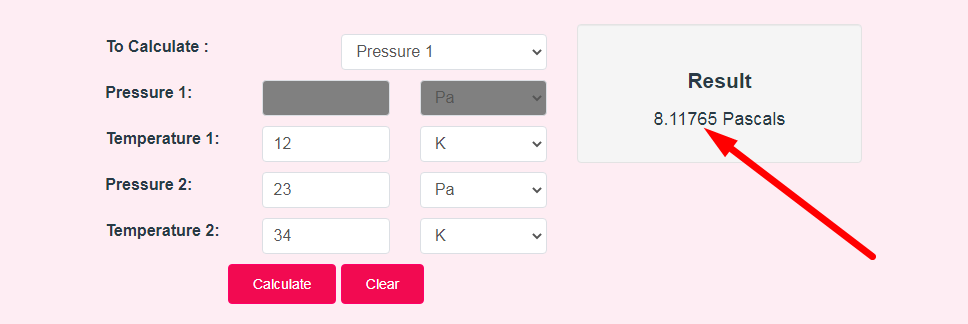
The free gay lussac’s law calculator helps you to do instant calculations for pressure and temperature of a gas enclosed in isochoric medium.
If you want to link to Gay Lussacs Law Calculator page, please use the codes provided below!
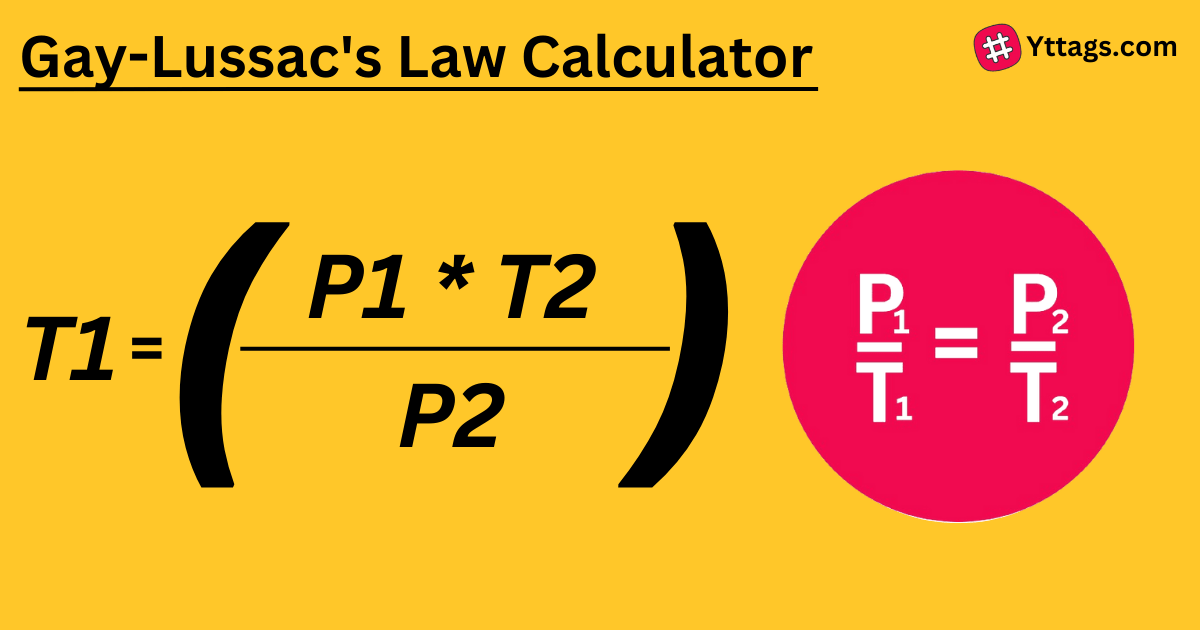
FAQs for Gay-Lussac's Law Calculator
What is a Gay-Lussac's Law Calculator?
A Gay-Lussac's Law Calculator is a tool used in thermodynamics to compute the final pressure or temperature of a gas when its volume is held constant, based on Gay-Lussac's Law, which describes the relationship between pressure and temperature.
What is the relationship between pressure and temperature equation?
The equations describing these laws are special cases of the ideal gas law, PV = nRT, where P is the pressure of the gas, V is its volume, n is the number of moles of the gas, T is its kelvin temperature, and R is the ideal (universal) gas constant.
What are the applications of gas law?
When the quantity of gas remains constant, but the pressure, volume, and temperature fluctuate, this rule applies. Cloud formation, refrigerators, and air conditioners, for instance, are all predicted by the law. It is also employed in computations involving thermodynamics and hydraulics.
What is the ideal gas law used for in real life?
Ideal Gas law has a lot more practical applications. It is being used to determine the densities of gases and in stoichiometric calculations. The coolants/refrigerants in your refrigerator, hot air balloons in the sky, and combustion engines in vehicles, all are based on the ideal gas law.
Is pressure proportional to temperature?
The pressure law states that for a constant volume of gas in a sealed container the temperature of the gas is directly proportional to its pressure.